Blog Post #6: Nanoparticles and Cancer Clinical Trials
This summer, I will be interning at a Clinical Trial Research Facility in Cincinnati, Ohio as a project coordinator intern, helping with clinical trials at all stages/phases. Therefore, when asked to explore the National Library of Medicine's comprehensive Clinical Trial database, I thoroughly searched the website. Before getting to the task at hand, I did search for Clinical Trials posted by the company that I will be working for in less than two weeks, Medpace. I found 65 studies done specifically by their Pharmacology unit; the company as a whole specializes in hematology, cardiology, and immunology. Medpace does a small amount of oncology research, but I could find nothing about nanoparticles. Therefore, I had to expand my search to connect oncology to nanoscience!
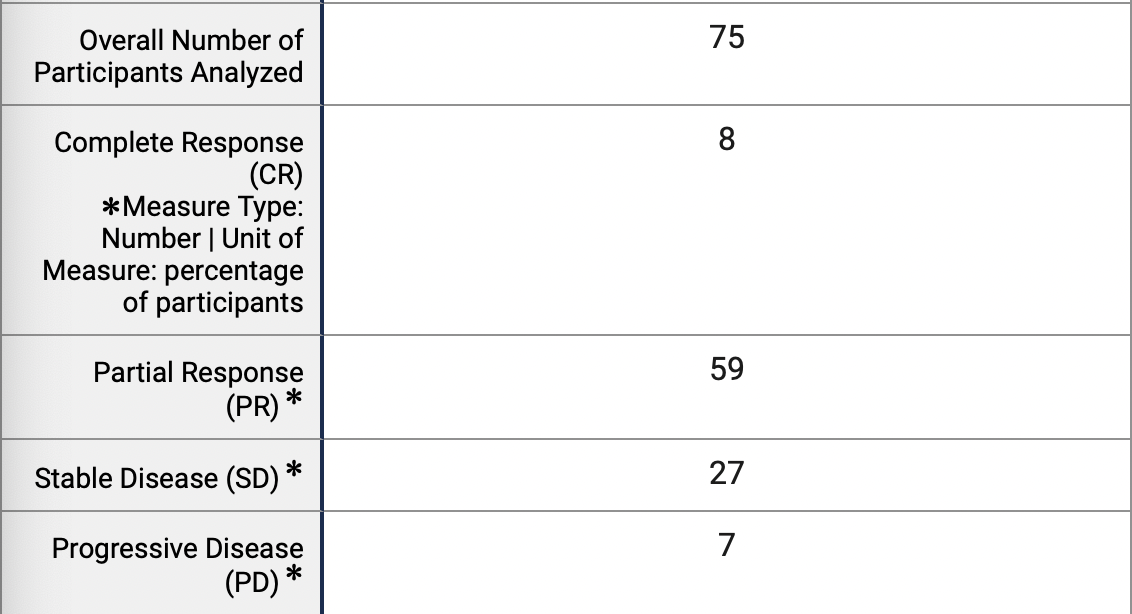
Instead of staying near Ohio (which I honestly kind of expected, the Ohio Valley Region has some of the top hospitals in the country), I found a clinical trial all the way in California testing Lung Cancer patients. With Lung Cancer, and some other cancers, some tumors are in precarious locations that cannot be removed with surgery. Therefore, this clinical trial was testing the capabilities of a compounded treatment of carboplatin and paclitaxel albumin stabilized nanoparticle formulation (then followed by radiotherapy and hydrochloride). Carboplatin and Paclitaxel are the names of a common chemotherapy combination, also called CarboTaxo and PC. Paclitaxel albumin-stabilized nanoparticle formulation is a specific mixture of paclitaxel that is packaged in protein nanoparticles. It is believed that this form of PC is more effective and has less side effects. From what I could research and understand, the albumin based nano protein carriers bypass drug efflux and enhance the drug uptake. So essentially, the nano proteins prevent the cancer cells from expelling the drug, meaning the drug stays in the system longer. I could not find as to why this would decrease side effects, other than maybe the drug spreading less to other parts of the body but chemotherapy is not cell specific. Despite this lack of understanding, I decided to look at the effectiveness and the side effects measured from the clinical trial done with the drug.
Works Cited:
“Carboplatin and Paclitaxel Albumin-Stabilized Nanoparticle Formulation Followed by Radiation Therapy and Erlotinib in Treating Patients With Stage III Non-Small Cell Lung Cancer That Cannot Be Removed By Surgery.” CTG Labs - NCBI, beta.clinicaltrials.gov/study/NCT00553462?tab=results. Accessed 18 May 2023.
Hassanin, Islam, and Ahmed Elzoghby. “Albumin-Based Nanoparticles: A Promising Strategy to Overcome Cancer Drug Resistance.” Cancer Drug Resistance (Alhambra, Calif.), 3 Nov. 2020, www.ncbi.nlm.nih.gov/pmc/articles/PMC8992568/#:~:text=Desolvation%20is%20the%20most%20common,the%20aqueous%20solution%20of%20albumin.





Comments
Post a Comment